From the chemical reactions of lithium-ion cells to the electricity that powers our everyday devices, understanding how battery cells work is essential for anyone trying to stay informed about today’s technology. In this article, we will explore the science behind batteries and discuss what makes them such a valuable form of energy storage.
Well, look at how they are made and why some types are more efficient than others, as well as explore some of their limitations. Finally, will examine some exciting developments in battery cell technology that could revolutionize our relationship with electricity in the years to come. Join us as we delve into the fascinating world of batteries and uncover exactly what makes them tick!
How Chemistry Affects Electricity Production
When it comes to electricity production, chemistry plays an integral role. Battery cells are the primary driving force behind this process, as they are composed of various elements and compounds that react with each other to generate electricity.
Through a chemical reaction known as oxidation-reduction, electrons move from one element or compound to another, creating a flow of current that can then be used for electricity production. This process is highly dependent on the type of chemicals being used; different combinations produce different levels and qualities of power output.
Furthermore, the temperature at which these reactions take place also has an impact on their efficiency – higher temperatures increase the speed at which energy is generated while lower temperatures reduce it. Ultimately, understanding how battery cells work requires a deep knowledge of both chemistry and physics to optimize electrical output for any given application.
Understanding the Components of a Battery Cell
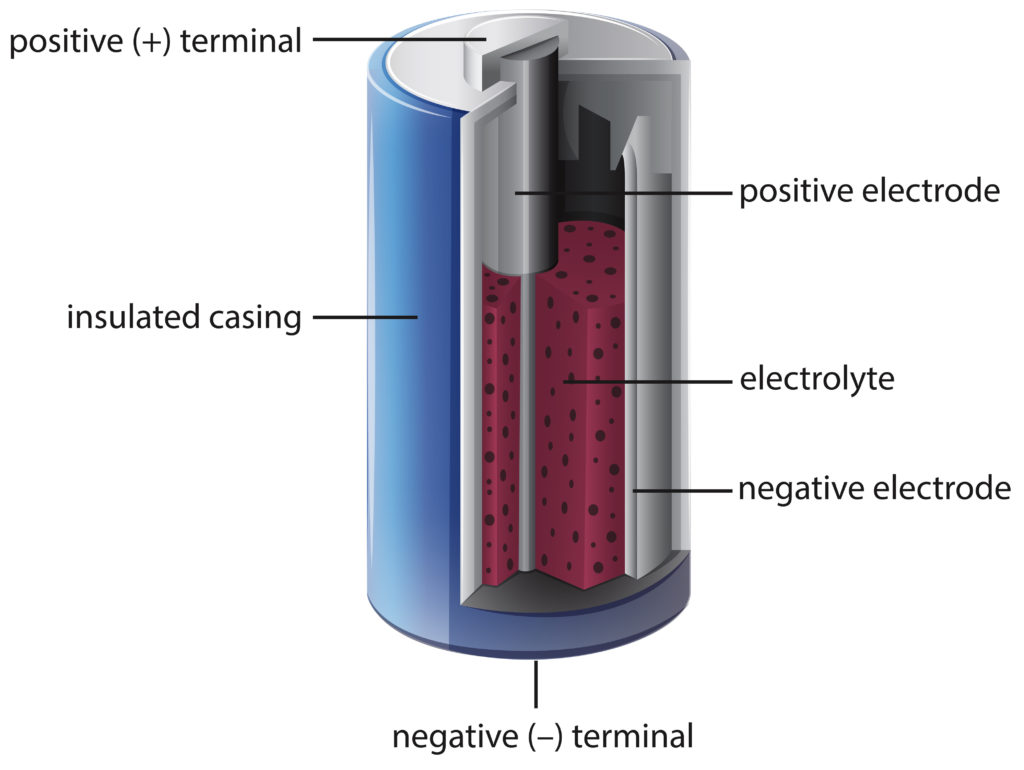
A battery cell is a complex device, but understanding it can be simplified by breaking down its components. The three primary elements of a battery cell are the electrolyte, anode, and cathode. The electrolyte acts as the medium for transferring ions between the two electrodes during charging and discharging while also providing mechanical support.
The anode serves to conduct electrons from outside into the system when in use and provides a space for oxidation reactions. Meanwhile, the cathode works to accept electrons from inside of the system when in use and provides a location for reduction reactions to take place.
Together these components work to convert chemical energy stored within them into electrical energy that can power various devices. Understanding how each component functions will give you greater insight into how batteries work on a fundamental level.
The Role of Electrochemistry in Generating Electricity
Electrochemistry is a powerful tool for generating electricity. It produces electric current and voltage by the movement of electrons between two electrodes immersed in an electrolyte solution.
This process, known as electrochemical redox reactions, occurs when two different chemical substances react with one another to create electrical energy. For this reaction to take place, both electrodes must be electrically connected.
Then, using an external source of power such as a battery or solar panel, the electrode will gain or lose electrons depending on whether it is positively charged (giving up electrons) or negatively charged (accepting them). As these reactions occur at either the anode or cathode side of the cell, electrical current flows through the circuit and generates usable electricity.
The use of electrochemistry has become increasingly important in powering modern technologies such as lithium-ion batteries found in mobile devices and laptop computers. By understanding how cells work from a chemistry perspective – from traditional lead acid batteries to newer technology like Lithium-Ion cells – we can design more efficient energy storage solutions that provide reliable power sources even under extreme weather conditions.
Therefore, with its ability to store large amounts of energy efficiently and safely within small spaces while producing clean electricity – Electrochemistry plays a vital role in providing renewable energy sources that are essential for our daily lives today.
Conclusion

Battery cells are a critical component of modern technology, enabling everything from laptops to electric cars. As their name implies, battery cells store and deliver electrical energy through chemical reactions that convert stored chemical energy into electricity.
By combining multiple individual cell units, engineers can create powerful batteries capable of powering all kinds of devices. Battery cells are complex pieces of technology that provide an essential service in the modern world and will continue to be improved upon for years to come.